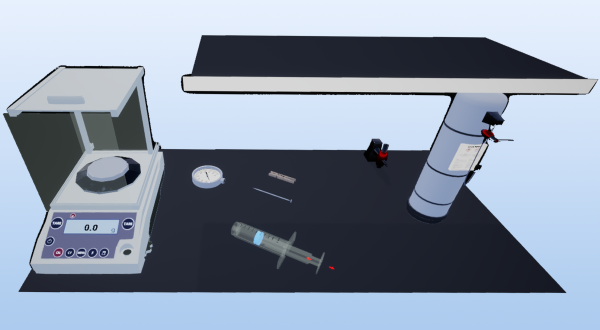
This laboratory session is designed to explore the relationship between the pressure and volume of a gas, employing a syringe and a dial pressure gauge for the experiment. The procedure involves attaching the syringe to an air cylinder and adjusting the air volume in the syringe to 55.0 ml. Subsequently, the syringe is connected to the dial pressure gauge in a waterproof seal, and the air volume is incrementally increased by 5.0 ml steps, with the pressure reading taken at each interval.
This experiment serves as a practical application of Boyle’s Law, which posits that the pressure of a gas is inversely proportional to its volume at a constant temperature.
Educational Goals
- Practical application of Boyle’s law: Participants will directly apply Boyle’s Law to understand the inverse relationship between gas pressure and volume.
- Precision in equipment handling: The session will teach students the accurate use of syringes and pressure gauges, emphasizing the importance of precision for reliable measurements.
- Observational and analytical skills: Students will enhance their skills in observing variations in pressure with changes in volume and analyzing these observations to confirm the validity of Boyle’s Law.
- Understanding gas thermodynamics: Through hands-on experimentation, participants will reinforce their conceptual knowledge of gas thermodynamics, particularly the principles governing the behavior of gases under varying pressures and volumes.
This laboratory provides participants with an invaluable opportunity to experiment with the principles of Boyle’s Law, reinforcing theoretical knowledge through practical application. By manipulating the syringe and pressure gauge to measure how gas pressure varies with volume, students gain a deeper understanding of gas behavior. This session not only improves their ability to handle laboratory equipment and collect data accurately but also deepens their comprehension of the fundamental concepts in the thermodynamics of gases, offering a solid foundation for further studies in physics and chemistry.
Protocol
- Pull the plunger of the syringe so that it contains exactly 50 mL of air.
- Connect the syringe to the dial gauge using the appropriate fittings. The assembly must be perfectly sealed and able to withstand high pressure.
- Pull on the piston to increase the volume by 10 mL. Read the pressure measurement.
- Repeat step 3 several times (at intervals of 5 ml) until reaching a volume of 100 ml.
- Hold the interaction button of the syringe to combat the pressure difference.
- Wait for the syringe to return to a volume of 50 mL and ensure that the pressure matches the one measured in step 3. If this is not the case, the experiment must be restarted.
- Push on the piston to reduce the volume by 10 mL. Read the pressure measurement and record the measurements in the results table.
- Repeat step 7 several times (at intervals of 5 ml) until reaching a volume of 20 mL.
- Hold the interaction button of the syringe to counter the pressure difference.
- Wait for the syringe to return to a volume of 50 mL and ensure that the pressure corresponds to that which was measured in step 3. If this is not the case, the experiment must be repeated.
Anticipated Outcomes
- Volume-pressure relationship (Boyle’s law): As the volume of the gas in the syringe increases, the pressure it exerts should decrease, and vice versa, illustrating an inverse relationship. This is expected to be observed as a continuous decrease in pressure readings on the manometer as the volume in the syringe is incrementally increased. The relation also follows the ideal gas law, where pV=nRT, where p= pressure (Pa), V=Volume (/1000L), n = moles, R = 8.314 J/mol*K, and T is the temperature in kelvins.
- Data pattern: Plotting the pressure against volume should yield a hyperbolic curve if the temperature remains constant, which is a graphical representation of Boyle’s Law. The product of pressure and volume at each point should be roughly constant, assuming ideal gas behavior.
- Accuracy and precision: The precision of the measurements, as well as the airtightness of the setup, are crucial for the validity of the results. Any leakage or measurement error could significantly skew the data, impacting the demonstration of the pressure-volume relationship.
Significance of the experiment:
- Understanding gas laws: This experiment is pivotal in reinforcing the conceptual understanding of gas laws, particularly Boyle’s Law, in a tangible and interactive manner. It bridges theoretical knowledge with practical application.
- Skills development: The procedure enhances technical skills such as precise measurement, operation of laboratory equipment (e.g., syringes and manometers), and data analysis, which are transferable to a wide range of scientific endeavors.
- Scientific reasoning: The experiment encourages analytical thinking by requiring students or participants to predict, observe, and rationalize the outcomes based on the principles of physics and chemistry.
- Safety and protocol adherence: Emphasizing safety and protocol adherence prepares participants for future laboratory work, underlining the importance of meticulousness and responsibility in scientific investigations.
This experiment, therefore, is not just a demonstration of a fundamental physical principle but also a comprehensive exercise in scientific methodology, critical thinking, and practical skills development, all of which are essential for proficiency in the scientific field.
Summary of Assignment by Grade Range
Grades 3-5 (Ages 8-10)
- Focus: Basic introduction to gas pressure and volume concepts.
- Activities: Observing changes in pressure and volume using simple demonstrations, basic safety instructions.
Grades 6-8 (Ages 11-13)
- Focus: Intermediate understanding of Boyle’s Law and gas behavior.
- Activities: Adjusting air volume in a syringe, measuring pressure with a dial pressure gauge, recording observations, following detailed safety protocols.
Grades 9-12 (Ages 14-18)
- Focus: Advanced understanding of Boyle’s Law, precise measurement techniques, and gas thermodynamics.
- Activities: Accurately adjusting and measuring air volume and pressure, analyzing the inverse relationship between pressure and volume, detailed recording and interpretation of results, adhering to advanced safety protocols, reinforcing concepts of gas behavior under varying conditions.
Laboratory essentials
Instruments
- Electronic scale
- Nail
- Syringe
- Wood clamp
Products
- Compressed air